The Dissolving Sentinels of the Southern Ocean
By Michael Lucibella, Antarctic Sun Editor
Posted March 24, 2016
Scientists are increasingly finding sea snails with abrasions all over their tiny spiral shells, but these scuffs aren’t just wear and tear. They’re ominous signs that a foundational link in the Southern Ocean’s food chain could be facing an existential crisis in waters that are becoming increasingly acidic.

Photo Credit: Michael Lucibella
Kevin Johnson and Juliet Wong pull a set of bongo nets out of the water. Each pull brings in thousands of the tiny pteropods.
The carbon dioxide that continues to build up in the atmosphere is changing the chemistry of the ocean and dissolving the shells of the Antarctic sea snails. Though not much bigger than a peppercorn, their small size belies their importance to the food web.
“A lot of people think of them as indicator organisms; harbingers of how an ecosystem might respond to a changing environment,” said Gretchen Hofmann, a professor at the University of California Santa Barbara (UCSB), whose research is supported by the U.S. Antarctic Program.
The National Science Foundation (NSF) manages the Antarctic Program, through which it funds scientists, coordinates all U.S. research on the continent and provides the logistical support that makes the science possible.
Hofmann and her team are engaged in a multifaceted effort to better understand the lifecycle of these snails, also known as pteropods. In particular, how they’re affected by the changing chemistry of the Ross Sea.

Photo Credit: Michael Lucibella
Kevin Johnson uses a pipette to separate out recently caught pteropods, each one not much bigger than a grain of sand
“Overall it’s really a project designed to look at the adaptive capacity and resilience of organisms living in the Southern Ocean,” Hofmann said.
Originally planned as a broad effort to better understand how vulnerable pteropods are in the early stages of their lifecycle, the research took on a new urgency last year, when members of the team were looking at the shells from specimens they collected in 2014. Instead of their expected smooth, pristine-looking surfaces, many appeared pitted and blotchy under the microscope.
Cailan Sugano, a research technician from UCSB, was making the observations under the microscope. He had previously been looking at the shells of scallops at the Woods Hole Oceanographic Institute that were dissolving in ocean water made artificially acidic from added carbon dioxide. The marks he saw on the pteropods looked worrying similar.
Dissolving Pteropod Shells Up Close

Images Courtesy: Cailan Sugano
Under an electron microscope, the center of a healthy shell is smooth and even
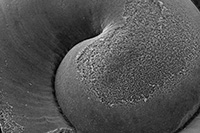
Elevated carbon dioxide levels in the oceans is already starting to dissolve many pteropod shells
“We looked closer and we found what was clearly dissolution,” Sugano said. “It’s sad and it’s surprising above everything else.”
When air mixes into the ocean, carbon dioxide from the air bonds with water molecules to form carbonic acid. The effects are twofold. First, it reduces the amount of carbonate minerals in the ocean for the pteropods to build into their shells. Additionally, the acid decreases the overall pH of the oceans, eating away the pteropods’ thin shells, made mostly of aragonite, a mineral particularly vulnerable to increased acidity.
The team was surprised by how widespread the damage was, and what it meant for the creatures’ ability to thrive wasn't clear. To better understand what was happening, part of the team flew to Antarctica during the period known as Winfly, at the end of August, before the station officially started its summer season. They hoped to catch young pteropods who had just survived their first winter under the ice shelf.
“We were surprised to see signs of dissolution on their shells already, so we wanted to see them younger,” Hofmann said. “If we got there at Winfly we could get a lot more science out of the project.”
As soon as the team reached McMurdo, they deployed a long, conical “bongo” net through a hole drilled in the ice in the sound. As clouds of pteropods carried by the underwater current floated by, the nets capture them by the thousands.

Photo Credit: Michael Lucibella
In addition to monitoring the harmful effects of acidifying oceans on pteropods, Kevin Johnson plans on studying their genome as well
“We had just literally hit the ground and within forty eight hours we were out in the field putting our net down for the first time,” said Kevin Johnson, a graduate student at UCSB. “We collected a lot of animals over WinFly.”
The team conducted a variety of experiments to understand the ocean chemistry itself, how much the pteropods’ shells were dissolving from the acidic oceans of today and the future, what it’s doing to their metabolisms and how that’s affecting their genomes.
Getting a baseline for how the ocean’s acidity changes over the year is a key component to their work. Hofmann’s team first deployed a series of automated ocean monitors around McMurdo Sound in 2010, providing what is now the longest continuous dataset on ocean pH in the region. It has helped them connect how the oceans are influenced by changes in the environment, and how pteropods are affected by changes in the ocean.

Photo Credit: Michael Lucibella
Juliet Wong returns the bongo nets to the ocean to troll for another sample of pteropods
“It’s sort of breaking down the walls of the fields. To do the biology right, you have to understand the chemistry of the ocean,” said Umihiko Hoshijima, who is also a graduate student at UCSB.
To quantify exactly how much pteropods are affected by the changing acidity around them, Sugano put together a chart to classify to what degree a creature’s shell has dissolved. While looking at their shells under an electron microscope, he ranks from 0 to 3 how damaged a section of a pteropod’s shell appears.
“The hardest thing about making that survey guide was finding ones without dissolution,” Sugano said. “We know these guys were sensitive to ocean chemistry, but we didn’t realize how sensitive.”
Previous 1 2 Next










