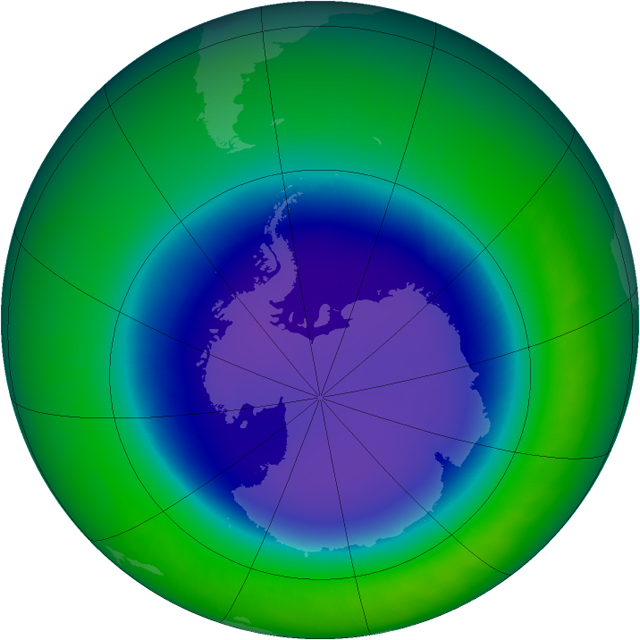
Ozone refresherPosted July 16, 2010
Ozone is a somewhat unstable molecule made up of three oxygen atoms instead of two. It’s found throughout the Earth’s atmosphere, with the highest levels occurring in the lower stratosphere, a region commonly referred to as the ozone layer, between about 10 and 30 kilometers above the planet’s surface. (The layer just below that is the troposphere, where we live and breathe.) The ozone layer blocks harmful ultraviolet rays that have been linked to skin cancer. The ozone hole over the Antarctic waxes and wanes every year between August and November as the Antarctic summer begins. (A similar but far smaller hole occurs above the Arctic during springtime there.) The hole is the result of the input of chlorofluorocarbons (CFCs) and other chlorine- and bromine-containing gases that interact with two naturally occurring events. One is the polar vortex, a sort of atmospheric cyclone that is strongest in winter when temperatures are below negative 80 degrees Celsius. The other is polar stratospheric clouds (PSCs), or nacreous clouds when visible, that also form in the extreme polar winter. The vortex creates a closed system that maintains extremely low temperatures and prevents the cold polar air from mixing with warmer air from the mid-latitudes. The ice crystals that form PSCs provide an excellent surface for setting chlorine and bromine free to run amok and destroy ozone in the presence of sunlight. So, the returning sun serves as the match to set this whole chemical pyre ablaze, beginning in August, breaking the free-floating chlorine molecules into their most reactive state. The ozone hole eventually “closes” as the vortex weakens and dissipates by November with the warming temperatures. The atmospheric mixing that ensues then consumes the harmful chlorine and bromine in other reactions, but also causes a small percentage of ozone depletion throughout the southern hemisphere as the depleted Antarctic air mixes with mid-latitude air. |



For USAP Participants |
For The Public |
For Researchers and EducatorsContact UsU.S. National Science FoundationOffice of Polar Programs Geosciences Directorate 2415 Eisenhower Avenue, Suite W7100 Alexandria, VA 22314 Sign up for the NSF Office of Polar Programs newsletter and events. Feedback Form |